Background: Within the heterogeneous genomic landscape of multiple myeloma (MM), clonal evolution including the acquisition of risk-defining mutations and chromosomal abnormalities is a recurrent event and can be detected by fluorescence in situ hybridization (FISH). The effects of acquired abnormalities on clinical outcomes have not been well defined. We previously reported that patients who acquired 17p deletion [del(17p)] during the course of their disease had a significantly reduced overall survival (OS) by 38 months compared to patients who did not acquire del17p (Lakshman et al 2019). Similarly, while de novo gain of the long arm of chromosome 1 (1q22 gain) is a known high-risk aberration associated with significantly shorter OS and progression-free survival (PFS) in MM, the prognostic significance of acquired 1q22 gain has not been described. The primary objective of this study was to analyze factors predictive for acquired 1q22 gain and determine its impact on survival.
Methods: We identified MM patients from the Mayo Clinic Dysproteinemia Database who had at least one follow up FISH performed ≥6 months from diagnosis. The clinical characteristics, concomitant cytogenetic abnormalities, first line treatments administered, and OS were compared between patients with acquired 1q22 gain and patients who did not acquire this abnormality. The Mayo Clinic IRB approved this study.
Results: A total of 1041 MM patients met the inclusion criteria. Of these, 63 patients (6.1%) had acquired 1q22 gain, defined as being negative for 1q22 gain on initial FISH at diagnosis and having this abnormality detected on subsequent FISH. Median age at diagnosis was 59 years and 56% were male. Median time to acquisition of 1q22 gain was 60 months (range 8-140 months). We identified one control patient for each case who was diagnosed within one year of the case and had a subsequent FISH performed at a similar duration from diagnosis. Patients with acquired 1q22 gain had similar baseline characteristics except for a higher proportion of high-risk (HR) FISH at diagnosis [t(4;14), t(14;16), t(14;20), and del(17p13)] when compared to controls (27% HR FISH versus 6% HR FISH; P<0.01). 1q22 gain was concomitantly present with trisomies in 33 patients (54%), monosomy 13 in 24 patients (39%), t(4;14) in 8 patients (13%), and del(17p13) in 7 patients (12%). All patients received treatment prior to acquisition of 1q22 gain. Of the 63 patients, first line induction therapy consisted of proteasome inhibitor (PI) with steroid in 43%, immunomodulatory drugs (IMiD) with steroid in 40%, and PI + IMiD with steroid in 17% of patients. 54 patients (85%) received upfront stem cell transplant (SCT) (median 5.9 months to SCT), compared to 50 patients (79%) in the control group who received SCT.
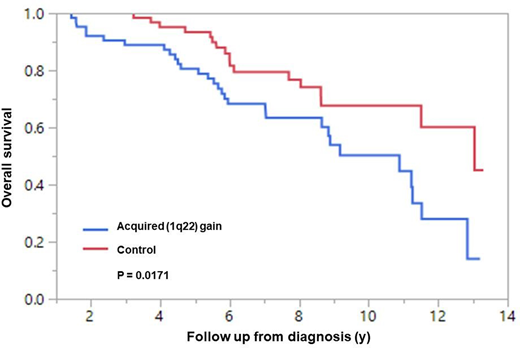
The median follow up of all 126 patients was 6.8 years. There was a statistically significant reduction in median OS from diagnosis in patients with acquired 1q22 gain compared to the control group (10.8 years versus 13.0 years; P = 0.02; Figure 1). Predictors of acquisition of 1q22 gain were identified using a case-control method. Age ≥70 at diagnosis and presence of HR FISH at baseline appeared to increase the risk of acquiring 1q22 gain.
Conclusion: Acquisition of 1q22 gain is a relatively uncommon occurrence, but notably reduced OS by 2.2 years compared to patients who did not acquire 1q22 during the course of their disease (P = 0.02). Older age and the presence of HR FISH at diagnosis increased the risk of acquisition of 1q22 gain. The presence of high-risk translocations at baseline suggests that acquisition of 1q22 gain occurs in the context of more aggressive disease biology.
Kapoor:Cellectar: Consultancy; Celgene: Honoraria; Janssen: Research Funding; Sanofi: Consultancy, Research Funding; Amgen: Research Funding; Takeda: Honoraria, Research Funding; GlaxoSmithKline: Research Funding. Dispenzieri:Alnylam: Research Funding; Pfizer: Research Funding; Celgene: Research Funding; Takeda: Research Funding; Intellia: Research Funding; Janssen: Research Funding. Gertz:Prothena: Consultancy; Celgene: Consultancy; Johnson and Johnson: Speakers Bureau; DAVA oncology: Speakers Bureau; Advisory Board for Pharmacyclics: Membership on an entity's Board of Directors or advisory committees; Advisory Board for Proclara: Membership on an entity's Board of Directors or advisory committees; i3Health: Consultancy; Springer Publishing: Patents & Royalties; Amyloidosis Foundation: Research Funding; International Waldenstrom Foundation: Research Funding; NCI SPORE MM: Research Funding; Ionis/Akcea: Consultancy; Physicians Education Resource: Consultancy; Medscape: Consultancy, Speakers Bureau; Amgen: Consultancy; Appellis: Consultancy; Annexon: Consultancy; Spectrum: Consultancy, Research Funding; Janssen: Consultancy; Sanofi: Consultancy; Data Safety Monitoring board from Abbvie: Membership on an entity's Board of Directors or advisory committees; Alnylam: Consultancy. Dingli:Apellis: Consultancy; Rigel: Consultancy; Millenium: Consultancy; Bristol Myers Squibb: Research Funding; Karyopharm Therapeutics: Research Funding; Sanofi-Genzyme: Consultancy; Alexion: Consultancy; Janssen: Consultancy. Lin:Novartis: Consultancy; Celgene: Consultancy, Research Funding; Bluebird Bio: Consultancy, Research Funding; Juno: Consultancy; Merck: Research Funding; Takeda: Research Funding; Gamida Cells: Consultancy; Sorrento: Consultancy, Membership on an entity's Board of Directors or advisory committees; Vineti: Consultancy; Legend BioTech: Consultancy; Janssen: Consultancy, Research Funding; Kite, a Gilead Company: Consultancy, Research Funding. Kumar:Cellectar: Other; Genecentrix: Consultancy; Dr. Reddy's Laboratories: Honoraria; AbbVie: Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments; BMS: Consultancy, Research Funding; Sanofi: Research Funding; Karyopharm: Consultancy; MedImmune: Research Funding; Takeda: Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments; Novartis: Research Funding; Kite Pharma: Consultancy, Research Funding; Oncopeptides: Consultancy, Other: Independent Review Committee; IRC member; Genentech/Roche: Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments; Celgene/BMS: Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments; Janssen Oncology: Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments; Adaptive Biotechnologies: Consultancy; Amgen: Consultancy, Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments, Research Funding; Merck: Consultancy, Research Funding; Carsgen: Other, Research Funding; Tenebio: Other, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.